March 15, 2024–Chorda Pharma today announced positive top-line results from a clinical trial for studying the skin burning characteristics of Capsadyn™ (0.25%), an over-the-counter topical analgesic formulated as a proprietary capsaicin-based cream with a concentration of 0.25%. Capsadyn™’s burning properties were statistically equivalent to the placebo cream (p = 0.32). On the other hand, Zostrix® (0.1%), an over-the-counter topical cream with a capsaicin concentration of 0.1%, had burning properties significantly higher than the placebo (p < 0.001).
Capsadyn™ is Chorda Pharma’s first non-opioid pain killer. The proprietary formulation is currently marketed in compliance with applicable regulations and policies of the FDA. The capsaicin concentration (0.25%) in Capsadyn™ is the maximum allowed by the FDA for over-the-counter topical analgesics.
Capsaicin is the molecule that makes chili peppers burn. The analgesic properties of capsaicin are well-known, but burning and irritation of the skin have limited the use of topical analgesics with capsaicin. The non-burning properties of Capsadyn™ make it unique among capsaicin-based topical analgesics.
“We believe that Capsadyn™ is very well positioned to address the growing market for relief of muscle and joint pain from arthritis and other conditions,” said Chorda Pharma’s CEO, Victor Iannello, ScD. “Capsadyn™ is non-addictive, it’s non-burning properties make it well-tolerated, and it’s safe to use with oral medications such as NSAIDs.”
Clinical Trial
The present study (Federal Registration: NCT05649228) used a double-blinded, randomized placebo-controlled trial to evaluate the potential for Capsadyn™ to induce dermal burning. Data were obtained from 44 healthy volunteers, and all participants completed the study. The clinical trial was conducted at a single site at the Dermatology Department, Carilion Clinic, Roanoke, Virginia.
The clinical trial studied the burning properties of three creams: Capsadyn™ (0.25%), Zostrix® (0.1%) as the active comparator, and a placebo cream with no capsaicin. For each participant, one cream was applied to one arm, and a different cream was applied to the other arm. The cream was externally applied to an area of 8 cm2 on the forearm and rubbed in until fully absorbed. Of the 44 participants, 30 participants had Capsadyn™ applied to one arm, 29 participants had Zostrix® applied to one arm, 29 participants had placebo applied to one arm.
After the creams were applied to a participant during a visit, a visual analog scale (VAS) was used for the participant to report the level of burning, where 0 represented no burning and 10 represented the highest level of burning. The monitoring lasted one hour with reports every 10 minutes. A follow-up telephone interview was conducted the day following the visit, and the maximum reported burn for the 24 hours after the visit was recorded.
Results
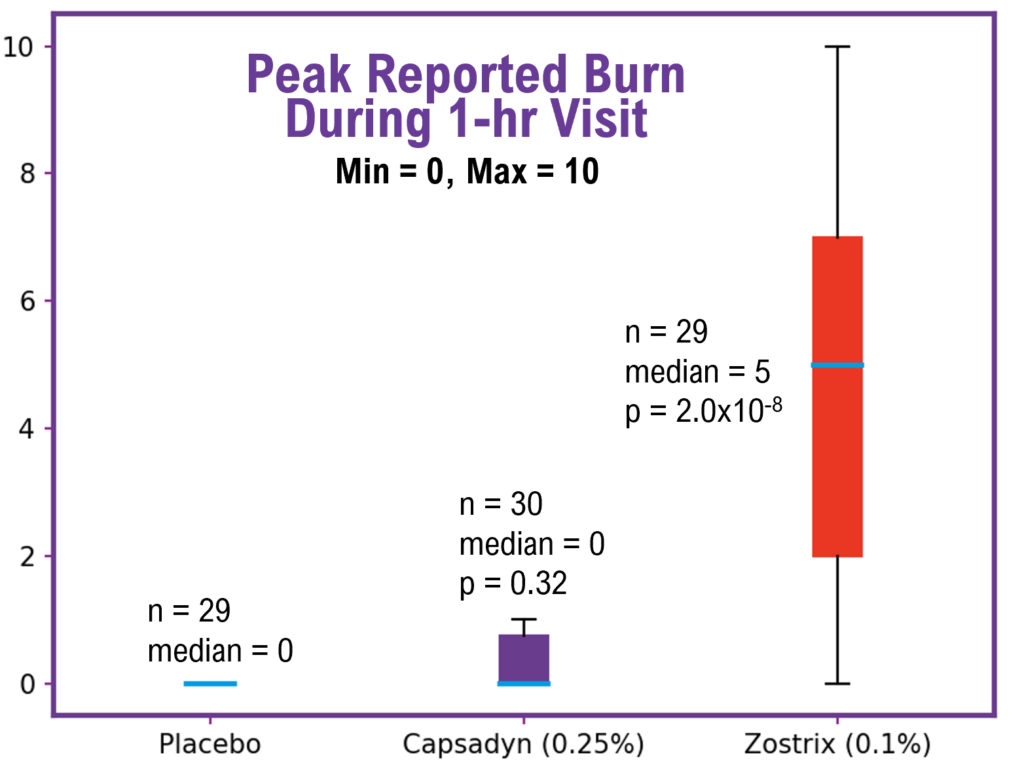
The first figure below shows the box chart results of the peak reported burn level during the one-hour visit. For the 29 participants with the placebo, the median burn level was 0. For the 30 participants with Capsadyn™, the median was also 0, and the burning level was statistically indistinguishable from the placebo (p = 0.32, Mann Whitney U). On the other hand, the participants with Zostrix® reported a median burn level of 5, with a statistically higher level of burn compared to the placebo (p < 0.001, Mann-Whitney U).
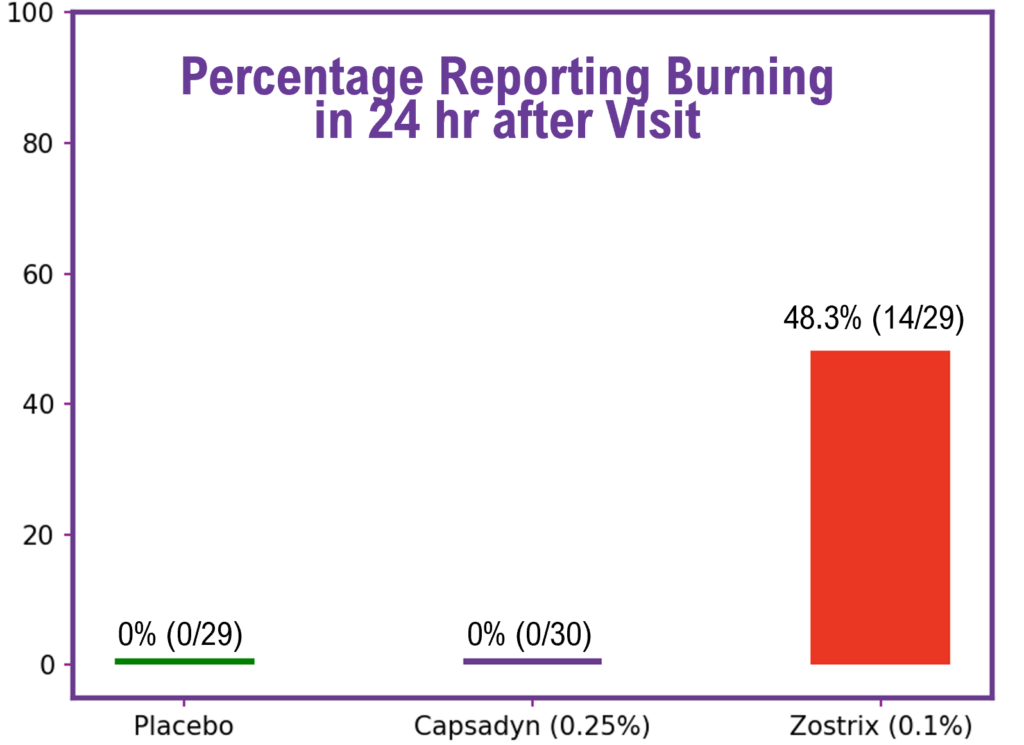
The next figure shows a bar chart of the burning level for each of the creams that was reported during the next-day interview. None of the participants with placebo (n = 29) or Capsadyn™ (n = 30) reported any burning. On the other hand, during the next-day interview, 48.3% (14/29) of the participants with Zostrix® reported burning during the 24-hour period following the visit.
The results from the clinical trial demonstrate that even at the maximum capsaicin concentration allowed by the FDA for over-the-counter topical analgesics, Capsadyn™ (0.25%) has statistically no difference in burning properties than a placebo. By comparison, Zostrix® (0.1%), with a concentration only 40% of Capsadyn™ (0.25%), has a statistically higher level of burning than a placebo.
Get Capsadyn™ Today for Pain Relief Without the Burn
The great news is that Capsadyn™ is now available for patients suffering from arthritis, muscle strains, sprains, bruises, related muscle injuries, and simple backache. Safe and effective for managing long-term pain, it’s now available in convenient 1-ounce jars, and will be shipped directly to you.